Introduction:
Classical Hodgkin lymphoma (cHL) is histologically composed of Hodgkin Reed-Sternberg (HRS) cells and the tumor microenvironment (TME) of the surrounding T-cells and macrophages, in which the expression of PD-1 and PD-L1 play critical roles in the immune response against cHL. Recently, multiplex immunofluorescence (MIF) staining and image analysis have enabled the evaluation of TME in cHL. However, the relationship between the characteristics of TME and clinical features of cHL is not fully understood. Therefore, we analyzed the pathology of patients with cHL and their clinical outcomes using MIF technology to evaluate the clinical features and the variations in TME.
Methods:
We retrospectively evaluated the clinical records and pathological samples for the diagnosis of patients with primary or relapsed cHL treated in our institution between 2010 and 2021. MIF staining and image analysis of TME were performed sequentially per the following process. Multiplexed immunofluorescence labeling against CD4, CD 8, CD30, CD68, CD163, PD-1, and PD-L1 was performed using the Opal 6-Plex Manual Detection Kit (Akoya Biosciences), and the image was captured using the Quantitative Pathology Workstation (Mantra2, Akoya). After image capture, the visual images were analyzed using supervised machine learning algorithms in Inform 2.6.0 (Akoya), which assigns phenotypes to all cells. To identify the structure of TME, the Euclidean distance from each HRS cell to other phenotype cell was calculated and identified, defined as area A within a radius of 30 µm from the HRS cells, area B from 30 µm to 60 µm, and area C from 60 µm to 120 µm. Thereafter, we analyzed the relationship between these parameters in TME at the initial diagnosis and the 3-year progression-free survival (PFS) calculated with Kaplan-Meyer methods. If the sample at the initial diagnosis was not available, the data from the sample at the first relapse were substituted.
Results:
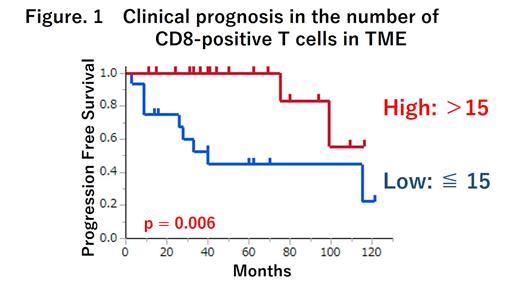
We obtained the data from 34 patients with 45 tissues, consisting of the pathological samples at the initial diagnosis (n = 32), the first relapse (n = 12), and the second relapse (n = 1). The median patient age was 46 years (range: 16-82). In the initial diagnosis, 18 and 16 patients had localized and advanced stages, respectively. All patients were initially treated with ABVD or BV-AVD therapy. During the median observation period of 61 months, 12 patients relapsed, and the 5-year PFS rate was 73%. All 45 tissues showed the expected patterns of cellular staining, with anti-CD30 delineating cells with HRS cells, anti-CD68 with macrophages (defined as anti-CD68-only expression as M1-macrophages (M1M) and co-expression of anti-CD68 and anti-CD163 as M2-macrophages (M2M)), and CD4 positive T-cells co-expressing anti-PD-1 and macrophages co-expressing anti-PD-L1. Comparisons of the characteristics of TME among areas A, B, and C revealed that CD8-positive T-cells, PD-1-positive CD4-positive T-cells, and PD-L1-positive macrophages were significantly more common in areas A and B than in area C, whereas PD-1 negative T-cells and PD-L1 negative macrophages exhibited no particular pattern in all areas. Thus, we identified that TME structures within 60μm are crucial for their immune effect on HRS cells. In the 34 patients at the initial diagnosis, we compared the TME within 60 µm and the PFS. As a result, TME containing higher numbers of CD8-positive T-cells (defined >15 cells) and M2M (defined > 13 cells) in TME were significantly associated with better 3-year PFS as compared with lower numbers of these immune components (100 vs 53%, p = 0.006 and 100 vs 65%, p = 0.012, respectively), whereas such findings were negative for CD4-positive T-cells or M1M. In the TME containing >15 cells of CD8-positive T-cell, significantly more M2M (mean 16 vs 5 cells, p=0.004) and fewer (mean 16 vs 28 cells, p=0.036) PD-1-positive T-cells were observed than in the TME containing ≤15 cells of CD8-positive T-cell. In the comparisons of 32 initial and 13 relapse samples, the number of CD8-positive T-cells TME significantly decreased at the time of relapse compared to the initial diagnosis (mean 7 vs 11 cells, p = 0.035).
Conclusion:
The findings of the MIF technology suggest that CD8-positive, cytotoxic T-cell enrichment in TME is a favorable prognostic factor for cHL. Such TME could be associated with PD-1/PD-L1-independent immune response against HRS cells. These results warrant further investigations.
Disclosures
Takahashi:Takeda: Honoraria; Chugai: Honoraria; Nippon Shinyaku: Honoraria; Sanofi: Honoraria; Kyowa Kirin: Honoraria; Janssen Pharma: Honoraria. Ito:Janssen Pharma: Honoraria. Miura:AstraZeneca: Honoraria; Chugai: Honoraria; Kyowa Kirin: Honoraria; Takeda: Honoraria; Bristol‐Myers Squibb: Honoraria; Nippon Shinyaku: Honoraria; SymBio: Honoraria; Ono Parma: Honoraria; Meiji Seika: Honoraria. Nakagawa:Bristol‐Myers Squibb: Honoraria; Janssen Pharma: Honoraria; abbvie: Honoraria. Nukariya:Chugai: Research Funding. Hatta:Kyowa Kirin: Honoraria; Janssen Pharma: Honoraria; Bristol‐Myers Squibb: Honoraria; Takeda: Honoraria; Ono Pharma: Honoraria; Chugai: Honoraria. Nakamura:MSD: Research Funding; Asahi Kasei Pharma: Research Funding; Astellas: Research Funding; AbbVie: Research Funding; Japan Blood Products organization: Research Funding; Eisai: Research Funding; Otsuka Pharmaceutical: Research Funding; Ono Pharma: Research Funding; Kyowa Kirin: Research Funding; Sanofi: Research Funding; Shionogi: Research Funding; Daiichi Sankyo: Research Funding; Taiho: Research Funding; Takeda: Research Funding; Mitsubishi Tanabe: Research Funding; Chugai: Research Funding; Teijin Pharma: Research Funding; Eli Lilly: Research Funding; Nippon Kayaku: Research Funding; Nihon Pharmaceutical: Research Funding; Boehringer Ingelheim: Research Funding; Pfizer: Research Funding; KALTECH Co.,Ltd: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal